There are at least six major applied fields of geoscience: oil, minerals, groundwater, unexploded ordnance, engineering geology, and environmental geology. There are others of course, but these are the main ones with corporate or government funding (as opposed to academic research, like geoarcheology).
Q: Where does gold come from?
– Kirk B.
A: Looking for gold deposits is a humbling experience for PhD geologists. That’s because there are uneducated people wearing little more than jeans, loin-clothes, or perhaps nylon shorts and flip-flops, who have already found them. That’s certainly the case historically in California, Venezuela, Saudi Arabia, and Africa where I have worked. King Solomon’s Ophir gold mines turned out to be 852 small ancient mines in the western Arabian Peninsula. Yes, the US Geological Survey worked there for 50 years at Saudi expense and actually counted them. At my house I have basalt and granite grind stones from one of them. These were used to crush quartz veins to free the tiny gold flakes used to make the fabulous items in King Solomon’s Temple. These stones are over 3,500 years old – they were dug up from beneath 5 meters (16 feet) of loess, dust blown across the Red Sea from the Sahara Desert in Africa. (Sahara, by the way, already means “desert” in Arabic, so Sahara Desert really means “desert desert.”) The miners, often slaves, painstakingly picked out each tiny gold flake with their fingers, one by one. When you read about King Solomon’s gold “basin” and huge gold Menorah, you are talking about millions of tiny flakes of gold, finger-picked one at a time, with the original ore extracted from tunnels often less than a meter high.
To have even a remote chance of finding any gold, perhaps the more important question is: how did gold get to where it is in the first place? Knowing the answer to this question can dramatically reduce the time and cost spent by a mining company searching for new deposits. If you spend $100,000,000 doing field exploration, drilling, building a mill, and hiring people to work in it – and you can only extract $10,000,000 in gold over 20 years – then your deposit is by definition not economic. You’ve wasted your investor’ money. The fact that every gold deposit is different from every other gold deposit also makes finding a new one even more difficult.
Studies over the centuries have shown that gold is about 5 times more abundant in rocks called “mafic” or “ultramafic” than in other types of rock. The crustal average value of a metal is called the “Clark” value. You have a good chance here of guessing who it was named after – or at least getting his last name right. Mafic and ultramafic rocks have more manganese (Ma) and iron (Fe), hence the “mafic” name, but this tiny Clark amount is still far less than the concentration level that would be economic to actually mine. Mafic rocks are generally darker than other igneous rocks, more magnetic, and very different from sedimentary rocks. They are often associated with magma produced at ocean-floor spreading centers, or the final phase of a multi-phase volcanic eruption field like what we see in southwestern Colorado or southeastern Arizona.
There must therefore be some sort of natural concentration mechanism, and we must be able to recognize the symptoms of this mechanism (for instance, altered or “stewed-looking” rocks), if we are going to have any chance of finding new gold deposits.
It turns out that a concentration mechanism, and the character of the final host rock, are equally as important as the source rock – if not more so. No matter what or where your source for diffuse gold is, the formation of a gold mine requires that concentrating mechanism. Studies suggest that this concentrating mechanism involves hot water, but a particular kind of hot water works best. The water must contain a lot of carbonate, a mineral related to the carbonic acid you drink in a soda, that is, carbon dioxide in solution in the water. Quartz veins with quartz and anchorite (something that looks like quartz but is somewhat creamy-colored, even brownish sometimes) host some of the best gold concentrations in Venezuela, North America, and elsewhere. Think of washing a vast volume of rock with hot Pepsi. Hey – it dissolves iron nails! Try dropping a nail into a bottle of Coke and checking on it a day or two later. You may stop drinking sodas…
Ah, but what gets this concentrating fluid moving? Typically, but not always, it is some sort of magma body emplaced in the early earth’s crust nearby: a big granite body coming from the mantle and intruded just below the surface will do very nicely. In Venezuela and the Arabian Shield, this is typically a hot crystalline mush, about 1 – 3 kilometers in diameter that punches up through the Earth’s crust like a fist. Another example is the Sierra Nevada granite batholith east of the Comstock Lode in California. The Comstock Lode is the roughly linear, north-south zone of mines west of the Sierra Nevada range. This “Mother Lode” drew the ’49’ers a century and a half ago to populate that state. Note the geographic association: the Comstock Lode lies west of and parallel to the huge, complex granite batholith that punched up through the earlier continental crust and makes up the Sierra Nevada range.
There are other mechanisms that can do the concentration, but try to visualize this: a big blob of magma, the top roughly 1-3 km across (far bigger in eastern California), grinds its way up from the upper mantle. The location if the intrusion favors weakened parts of the earlier crust that have been fractured by earlier faulting. If you try to punch your fist up through a mattress, it’s easier where the mattress is already torn. The intrusive body may have floated up from partial melting of a down-going slab of oceanic crust, as the North American continental plate slid westward over that oceanic crust. By partial melting, I mean that there is a partial segregation of the lighter, more silica-and-fluid-rich parts of the down-going oceanic crustal plate – and “silica-rich” is a defining characteristic of granite. Many examples of this can be seen today in the Andes, much of western North America and southern Alaska. This body of hot, viscous crystal-rich mush may or may not reach the Earth’s surface. It may be mostly or completely buried in the older, cold crust – and there it slowly cools underground to crystalize into a granite. The slower it cools, the bigger the crystal size of the individual grains in the granite. This cooling may take up to a million years, and is mainly carried out by convective flow of the local ground-water, not unlike what happens in your kitchen sauce-pan when you cook your Cream of Wheat. Only think of cooking your Cream of Wheat in hot Pepsi, with the carbonates coming from, say, a limestone body somewhere along the way. Limestone is a sedimentary rock made up of the calcium-carbonate shells of tiny, long-dead marine animals.
The convective flow patterns you see in Cream of Wheat and in gold concentration processes are called hydrothermal cells. For gold these cells can be vast in size, stretching more than 10 kilometers across. Typically, distant ground water, present nearly everywhere in the shallow Earth’s crust, is drawn towards the heat source. In the process it leaches out gold and other minerals (including carbonates) from vast volumes of surrounding rock along the way.
As the hydrothermal (literally, “hot water”) fluid approaches the magma, it heats up and expands into the broken-rock fractures caused by the upwelling magma body. As the fluids rise in the cracks and fractures and gets closer and closer to the surface, there is a reduction in the overlying rock pressure, which then sends these fluids racing towards the surface even faster as dissolved gases expand. It’s also cooling down as it does this, and anyone who took high school chemistry knows that cooling is one way to get things to precipitate out of solution into crystalline form. You’ve probably seen this as the white guck that falls to the bottom of your freshman chemistry test-tube. Now think back to a hike when you were younger and you can probably remember veins of white stuff criss-crossing some of the rocks. Same thing.
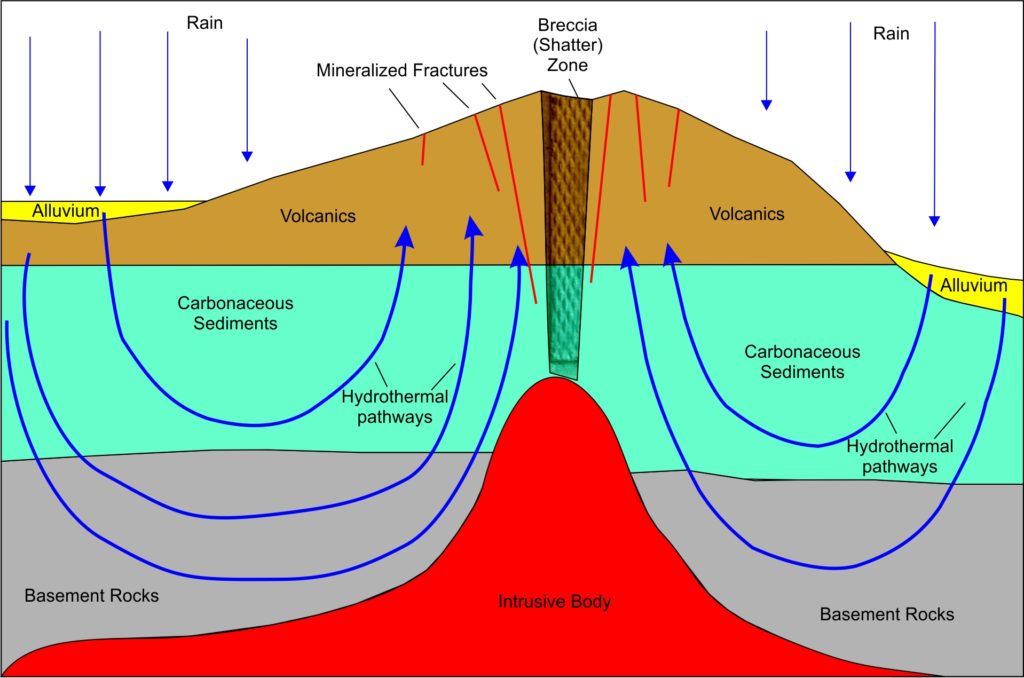
In the meantime, the intrusion of the magma body – that hot crystalline mush monster coming up from the Mantle – has caused the rocks above and around it to fracture and pulverize. A shattered rock zone is called a “breccia”, by the way – an Italian word from where it was first described. Some rocks are more brittle than others, shatter more easily, and thus are better hosts for mineral deposition; ancient volcanic rocks are particularly good for this.
In Australia, Canada, Venezuela and California there is a much higher incidence of gold deposits in ancient volcanic rocks punched through by newer magma bodies. The upwelling, mineral-laden hot water finds these fractures, and complex physical and chemical reactions take place. Since pressure and temperature drop rapidly in the fractures as fluids approach the surface, minerals will precipitate out on the walls of the fractures, eventually filling in towards the center of the fractures until the system seals itself shut. The fractures have now been converted to “veins” of quartz, anchorite, and other minerals (and maybe gold if there was a good mafic source rock somewhere along the hydraulic path the fluids took). Often there are other minerals, such as arsenic and silver, which tend to have a similar mobility to that of gold in these types of solutions. This same mobility and concentration process is the reason for arsenic, silver, mercury, and other minerals to form halos surrounding many gold deposits. Find the faint arsenic halo, and you can home in on the gold at the center. That’s one reason why mining companies hire chemists.
So far, I have described just one type of primary gold deposit, and there are others, such as the huge Carlin Trend in Nevada, closely associated with mercury, and the giant Homestake mine in South Dakota. There are secondary gold deposits however, and these are called placers. A town on the edge of the Mother Lode in California is called “Placerville.” Placers are where the gold and quartz have been weathered out of their original concentration points and have been carried down rivers. The Sacramento and Yukon Rivers are famous sources of placer gold nuggets. If you want to look for gold in a stream or river, look to the inside of a kink or curve in the river. Heavy minerals including gold and platinum group metals tend to drop out on the inside of the curve, where the current is slower, and has less energy.
Because gold and platinum-group metals are so-called “noble” elements, they don’t readily react to acids and oxygen, and when they accumulate, they will not dissolve and weather away like pyrite.
This is the academic story of gold deposits. The US Geological Survey, over more than a century, has assembled a book of ore deposit models, describing their distinct “signatures” to help other exploration geologists work more efficiently (Cox and Singer, 1986). There are countless other volumes on ore deposit geology, but these will get you started: Lindgren, W., 1933; Guilbert and Park, 1986; Edwards and Atkinson, 1986.
History, however, has shown that it’s not the PhD geologist who finds the gold deposits first, but the lone hungry prospector with a gold pan. This means anyone can find gold – but if the creek or quartz-rich ridge near you have already been walked over by humans, any gold has probably already been found. And claim-staked.
Don’t let that discourage you, though!
